The RASTA-AF study has 16 participating sites across Canada.

Participating Sites
| Halifax QEII Health Sciences Centre | Foothills Hospital Calgary |
| Regina General Hospital | St. Paul’s Vancouver |
| Kelowna General Hospital | Saint John Reginal Hospital |
| Hamilton Health Sciences Centre | Sunnybrook Health Sciences Centre |
| St. Michael’s Hospital | Ottawa Heart Institute |
| London Health Sciences Centre | St. Mary’s General Hospital |
| McGill University Health Centre | Montreal Heart Institute |
| Hôpital Laval | University of Alberta Hospital |
Clinical Question:
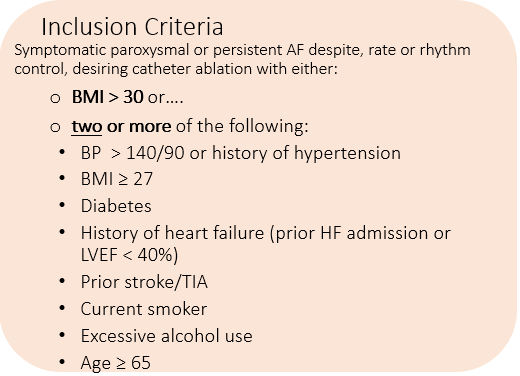
In patients with symptomatic Atrial Fibrillation (AF) and risk factors that promote AF, will vigerous treatment with a home-based exercise and risk factor modification program in combination with catheter ablation reduce AF-related outcomes compared to usual care?
Study documents
All required study documents can be found in the most recent version of the Reference Manual (dated Aug, 2023), including:
- Protocol version 15.1
- Device instructions
- Patient teaching materials
- Schedule of Activities
- Case report forms
- Patient questionnaires
Email RastaAF@nshealth.ca if you require a copy
Confirm-Rx ICM
Each study patient will receive a Confirm-Rx or Jot DX insertable cardiac monitor (ICM) from Abbott Medical Canada.
Sites will submit a no-cost purchase order for the RASTA Clinical Study for the devices (DM3500) on consignment. As they are implanted, they will be billed out and replaced at no charge. De-identified study data will be transmitted daily via the myMerlin smartphone app. Each site will have access to their patient’s data via the Merlin.net Patient Care Network.
If an ICM becomes disconnected from Merlin.net, patients can reach out to myMerlin Customer Support (1-877-696-3754) with their Confirm Rx serial number for support.



Home-based Rehabilitation Program
Patients randomized to aggressive risk factor management will complete 10-14 weeks of a structured counseling program delivered via weekly phone calls from a certified clinical exercise physiologist mentor. Topics will include physical activity, nutrition and stress management, with goal setting tailored to the patient and their environment.
The program has been developed and validated by Dr. Jennifer Reed at the University of Ottawa Heart Institute and can be delivered to both English (Ottawa Heart) and French ( Pavillon de Prévention des Maladies Cardiaques) speaking participants.

Home-based Sleep Testing
Participants in the risk factor management group, who do not have a sleep apnea diagnosis, and haven’t had a sleep study in the las 12-months, will be loaned an Alice NightOne device.
Sites will be provided 1-2 Alice NightOne devices, which come with all required accessories (e.g., batteries, per patient use nasal cannulas, USB cord for data upload). Each patient will take the device home and use it until a successful study is recorded, we recommend a minimum of 2-nights study; the device has 4 GB of memory and the average study size is 50 MB. The device is returned to the study coordinator and the largest file uploaded to the study database. See Device Instructions in your Reference Manual for complete details.

Our Partners





